- 13035986501
- 13035986501
- Nyx-peptide@jsjpharm.cn
Your Location:Home >Products >Small molecular peptide >45170-31-8
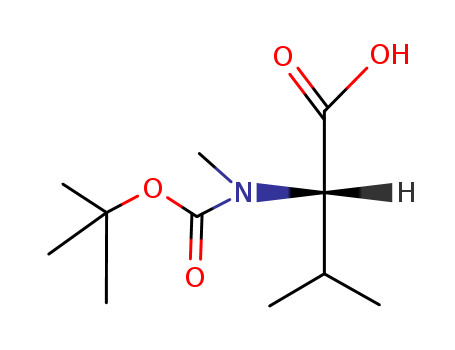

Product Details
| Description |
Boc-N-Me-Val serves as a versatile compound with applications in pharmaceutical intermediates, organic synthesis, and the construction of potential antitumor chemotherapeutic agents. Its role in peptide synthesis, protected by the Boc group, makes it valuable for controlled reactions in research and various chemistry applications. |
|
Chemical Properties |
White powder |
|
Uses |
Widely used in laboratories for synthesizing custom peptides, particularly in peptide chemistry research. |
InChI:InChI=1/C11H21NO4/c1-7(2)8(9(13)14)12(6)10(15)16-11(3,4)5/h7-8H,1-6H3,(H,13,14)/t8-/m1/s1
Triostin A, a cyclic octadepsipeptide, w...
Hoshinoamides A, B and C, linear lipopep...
PROBLEM TO BE SOLVED: To provide: a meth...
Kinenzoline (1), a new linear depsipepti...
Provided is a conjugate of a cytotoxic d...
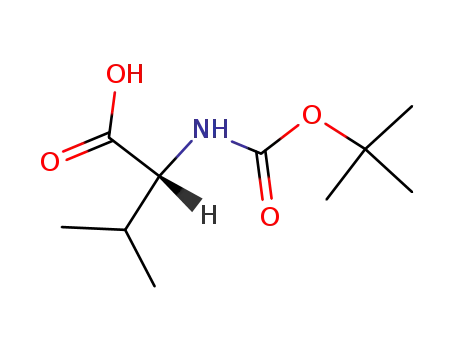
Boc-D-Val-OH

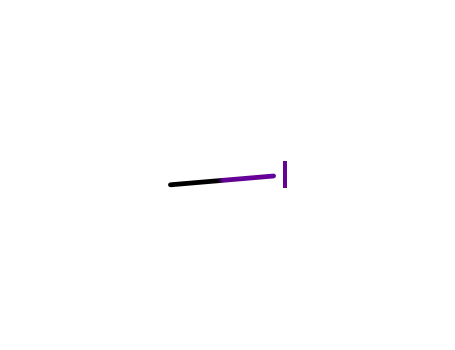
methyl iodide

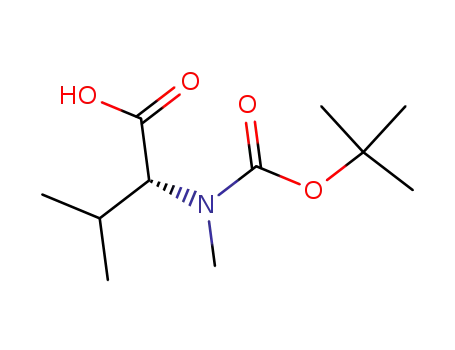
(R)-2-(tert-butoxycarbonyl(methyl)amino)-3-methylbutanoic acid
| Conditions | Yield |
|---|---|
|
Boc-D-Val-OH; With sodium hydride; In tetrahydrofuran; mineral oil; at 0 ℃; for 0.5h; Inert atmosphere;
methyl iodide; In tetrahydrofuran; mineral oil; at 20 ℃; for 36h; Inert atmosphere;
|
100% |
|
With sodium hydride; In tetrahydrofuran; at 20 ℃; for 24h;
|
87.2% |
|
Boc-D-Val-OH; methyl iodide; With sodium hydride; In tetrahydrofuran; mineral oil; at 0 - 20 ℃; Inert atmosphere;
With hydrogenchloride; In water; pH=2;
|
84% |
|
With sodium hydride; In tetrahydrofuran; mineral oil; at 20 ℃; for 24h;
|
80% |
|
With sodium hydride; In tetrahydrofuran;
|
|
|
With sodium hydride; In tetrahydrofuran; at 20 ℃;
|
|
|
With sodium hydride; In tetrahydrofuran; mineral oil; at 0 - 20 ℃; for 18h;
|
|
|
With sodium hydride; In tetrahydrofuran; at 20 ℃;
|
|
|
Boc-D-Val-OH; methyl iodide; In N,N-dimethyl-formamide; at 0 ℃; for 0.75h;
With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 20 ℃; for 5h;
|
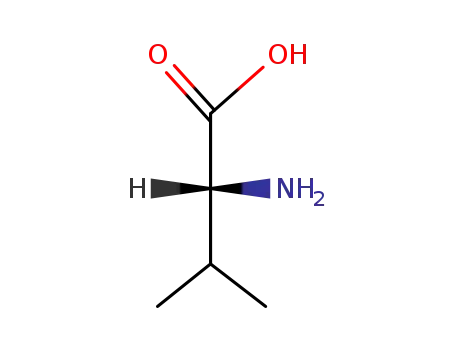
D-Val-OH

di-tert-butyl dicarbonate


methyl iodide


(R)-2-(tert-butoxycarbonyl(methyl)amino)-3-methylbutanoic acid
| Conditions | Yield |
|---|---|
|
D-Val-OH; di-tert-butyl dicarbonate; With sodium hydroxide; In 1,4-dioxane;
methyl iodide; In tetrahydrofuran;
|
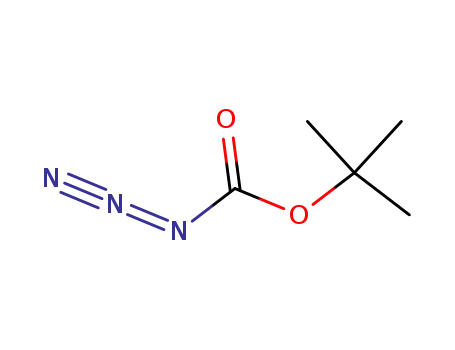
N-(tert-butyloxycarbonyl) azide
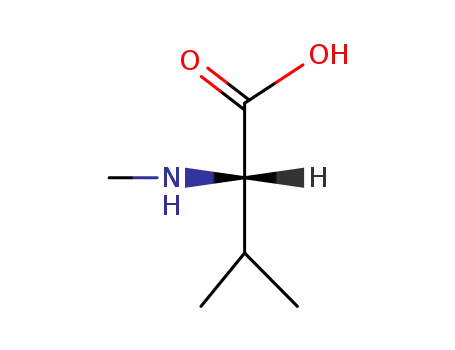
N-methyl-L-valine
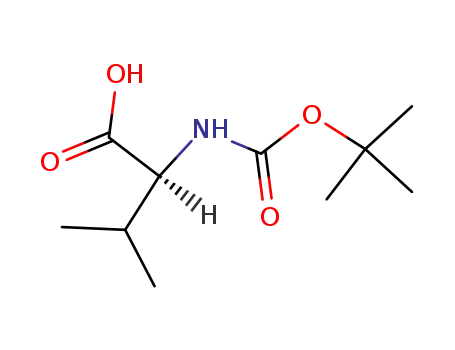
t-Boc-L-valine

methyl iodide
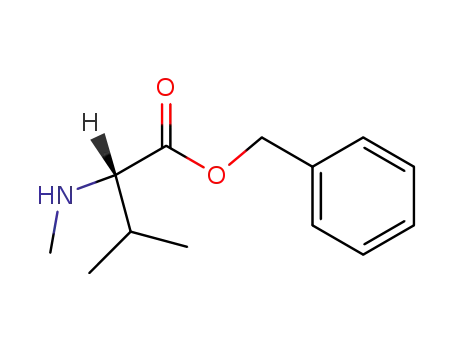
benzyl (2S)-3-methyl-2-(methylamino)butanoate
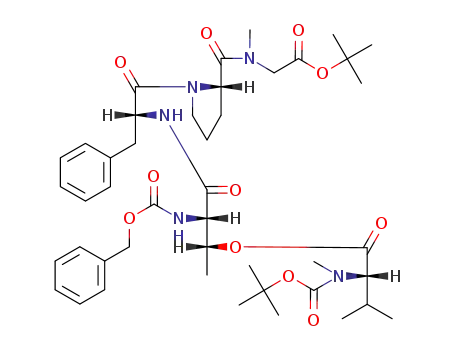
O-(tert-butoxycarbonyl)-N-methylvalyl-N-(benzyloxycarbonyl)threonyl-D-phenylalaninylprolylsarcosine tert-butyl ester
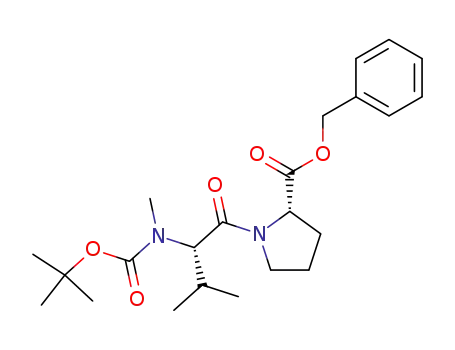
(S)-benzyl 1-((S)-2-(tert-butoxycarbonyl(methyl)amino)-3-methylbutanoyl)pyrrolidine-2-carboxylate
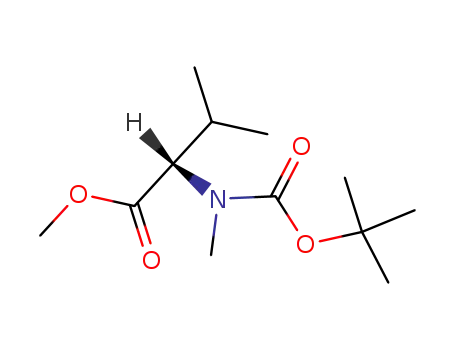
methyl (2S)-2-[(tert-butoxycarbonyl)(methyl)amino]-3-methylbutanoate
CAS:247062-33-5
CAS:79561-22-1
CAS:47931-85-1
CAS:120287-85-6
