- 13035986501
- 13035986501
- Nyx-peptide@jsjpharm.cn
Your Location:Home >Products >Custom peptide >120287-85-6

Product Details
|
Description |
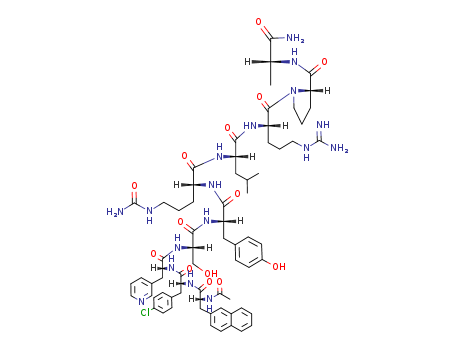
Cetrorelix Acetate, also known as SB-75 acetate, is a potent gonadotropin-releasing hormone (GnRH) receptor antagonist with an IC50 of 1.21 nM. It is utilized in the treatment of female infertility, particularly in controlled ovulation during in-vitro fertilization (IVF) procedures. Cetrorelix is a decapeptidic analog of luteinizing hormone-releasing hormone (LH-RH) with structural modifications at crucial positions. Notably, it bears the designation [Ac-D-Nal1, D-4-CI Phe-2, D-Pal3, D-Cit6, D-Alal0]-GnRH. |
|
Originator |
Asta Medica (Germany) |
|
Uses |
This drug acts as an extremely potent and long-acting GnRH antagonist, leading to the immediate blockade of gonadotrophins and sex steroid secretion upon administration. Additionally, it exhibits low histamine-releasing potency. Cetrorelix is distinguished as the first LH-RH antagonist approved worldwide. In Phase III clinical trials, female patients receiving Cetrorelix experienced successful controlled ovulation, preventing premature LH-surge. This positions Cetrorelix as a potential first-choice treatment for IVF, offering advantages over current controlled ovarian hyperstimulation protocols. |
|
Brand name |
Cetrotide (Serono). |
InChI:InChI=1/C70H92ClN17O14/c1-39(2)31-52(61(94)82-51(15-9-28-77-69(73)74)68(101)88-30-10-16-58(88)67(100)79-40(3)59(72)92)83-60(93)50(14-8-29-78-70(75)102)81-63(96)54(34-43-20-25-49(91)26-21-43)86-66(99)57(38-89)87-65(98)56(36-45-11-7-27-76-37-45)85-64(97)55(33-42-18-23-48(71)24-19-42)84-62(95)53(80-41(4)90)35-44-17-22-46-12-5-6-13-47(46)32-44/h5-7,11-13,17-27,32,37,39-40,50-58,89,91H,8-10,14-16,28-31,33-36,38H2,1-4H3,(H2,72,92)(H,79,100)(H,80,90)(H,81,96)(H,82,94)(H,83,93)(H,84,95)(H,85,97)(H,86,99)(H,87,98)(H4,73,74,77)(H3,75,78,102)/t40-,50-,51+,52+,53-,54-,55+,56-,57-,58?/m1/s1
-
The invention relates to methods for the...
Ac-D-Nal-D-Cpa-D-Pal-Ser(tBu)-Tyr(tBu)-D-Cit-Leu-Arg(Pbf)-Pro-D-Ala-NH2

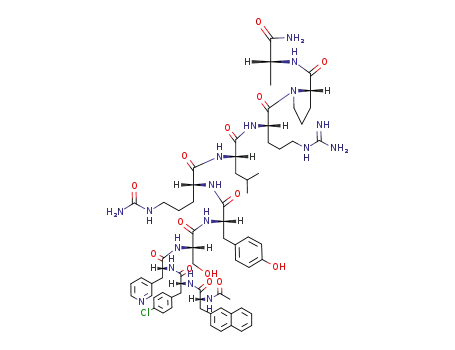
cetrorelix
| Conditions | Yield |
|---|---|
|
With chlorotriisopropylsilane; ethane-1,2-dithiol; trifluoroacetic acid; at 20 ℃; for 2h;
|
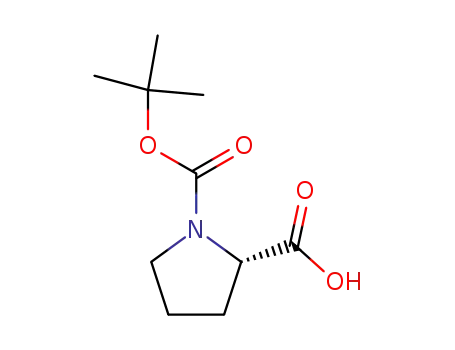
1-(tert-butoxycarbonyl)-L-proline

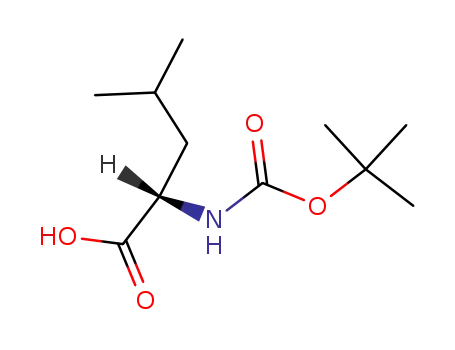
N-tert-butoxycarbonyl-L-leucine

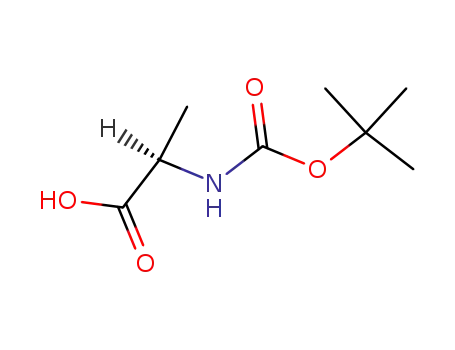
Boc-(R)-Ala

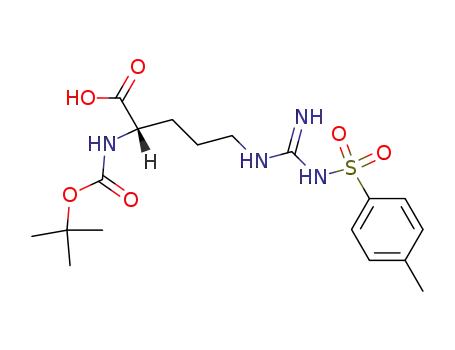
Boc-Arg(Tos)-OH


cetrorelix
| Conditions | Yield |
|---|---|
|
Multistep reaction;
|

1-(tert-butoxycarbonyl)-L-proline

N-tert-butoxycarbonyl-L-leucine

Boc-(R)-Ala

Boc-Arg(Tos)-OH
CAS:57022-38-5
CAS:247062-33-5
CAS:45170-31-8
CAS:188627-80-7
