- 13035986501
- 13035986501
- Nyx-peptide@jsjpharm.cn
Your Location:Home >Products >Custom peptide >188627-80-7

Product Details
|
Biological Activity |
Eptifibatide is a potent glycoprotein IIb/IIIa antagonist (GPIIb/IIIa; Kd = 120 nM) that inhibits platelet aggregation. Eptifibatide prevents binding of the adhesion proteins fibrinogen and von Willebrand factor to GPIIb/IIIa on the surface of activated platelets to prevent aggregation and thrombus formation. It inhibits ADP-induced citrated blood aggregation (IC50 = 0.11-0.22 μg/ml) in vitro and in vivo (IC50 = 52 μg/ml in porcine plasma). Formulations containing eptifibatide have been used to reduce risk of thrombolysis in myocardial infarction in patients undergoing percutaneous coronary intervention. |
|
Mechanism of action |
Eptifibatide, a synthetic cyclic heptapeptide containing six amino acids, including one cysteine amide and one mercaptopropionyl (desamino cysteinyl) residue, is an inhibitor of platelet aggregation and belongs to the class of RGD (arginine-glycine-aspartate)-mimetics.Eptifibatide reversibly inhibits platelet aggregation by preventing the binding of fibrinogen, von Willebrand factor and other adhesive ligands to the glycoprotein (GP) IIb/IIIa receptors. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Iloprost: increased risk of bleeding. |
|
Metabolism |
Renal excretion accounts for approximately 50% of total body clearance of eptifibatide; approximately 50% of the amount cleared is excreted unchanged in the urine. |
|
|
|
|
Definition |
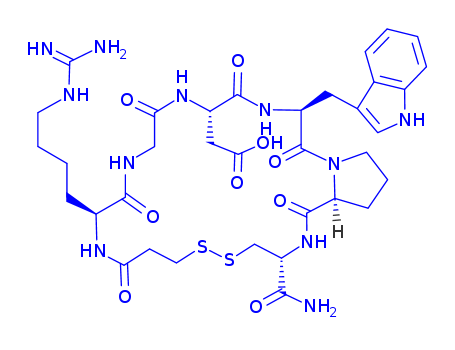
ChEBI: Eptifibatide is a synthetic homodetic cyclic peptide comprising N(alpha)-(3-sulfanylpropanoyl)homoarginyl, glycyl, aspartyl, tryptophyl, prolyl and cysteinamide residues connected in sequence and cyclised via a disulfide bond. Derived from a protein found in the venom of the southeastern pygmy rattlesnake, Sistrurus miliarus barbouri, eptifibatide is an anti-coagulant that inhibits platelet aggregation by selectively blocking the platelet glycoprotein IIb/IIIa receptor, so preventing the binding of fibrinogen, von Willebrand factor, and other adhesive ligands. It is used in the management of unstable angina and in patients undergoing coronary angioplasty and stenting procedures. It has a role as a platelet aggregation inhibitor and an anticoagulant. It is an organic disulfide, a macrocycle and a homodetic cyclic peptide. |
|
Brand name |
Integrilin |
|
General Description |
Eptifibatide (Integrilin) is a syntheticcyclic heptapeptide that acts as a GPIIb/IIIa receptor antagonist,thus causing inhibition of platelet aggregation. Itsstructure is based on the natural product barbourin, a peptideisolated from the venom of a pygmy rattlesnake (Sistrurusmilarud barbouri). As part of the structure, there is a sequenceRGD that can bind to the RGD receptor found onplatelets and block its ability to bind with fibrinogen. Thisagent is used in the treatment of unstable angina and for angioplasticcoronary interventions. |
InChI:InChI=1/C35H49N11O9S2/c36-31(52)26-17-57-56-12-10-27(47)43-22(7-3-4-11-39-35(37)38)32(53)41-16-28(48)44-25(14-29(49)50)34(55)45-24(13-18-15-40-20-6-2-1-5-19(18)20)30(51)21-8-9-23(42-21)33(54)46-26/h1-2,5-6,15,21-26,40,42H,3-4,7-14,16-17H2,(H2,36,52)(H,41,53)(H,43,47)(H,44,48)(H,45,55)(H,46,54)(H,49,50)(H4,37,38,39)/t21?,22-,23-,24-,25-,26-/m0/s1
The present invention provides processes...
An efficient method for the synthesis of...
Mpa-Har-Gly-Asp-Trp-Pro-Cys-NH2

integrilin
| Conditions | Yield |
|---|---|
|
With ammonium hydroxide; In water; acetonitrile; at 23 ℃; for 2h; pH=9;
|
63% |
di-isopropyl ether

chlorotriisopropylsilane

integrilin
| Conditions | Yield |
|---|---|
|
With trifluoroacetic acid; In dichloromethane; water; ethyl acetate;
|
|
|
With trifluoroacetic acid; In dichloromethane; water; ethyl acetate;
|
Mpa-Har-Gly-Asp-Trp-Pro-Cys-NH2
di-isopropyl ether
chlorotriisopropylsilane
eptifibatide acetate
CAS:40077-57-4
CAS:47931-85-1
CAS:120287-85-6
CAS:1392278-76-0
