- 13035986501
- 13035986501
- Nyx-peptide@jsjpharm.cn
Your Location:Home >Products >Custom peptide >60117-17-1

Product Details
|
General Description |
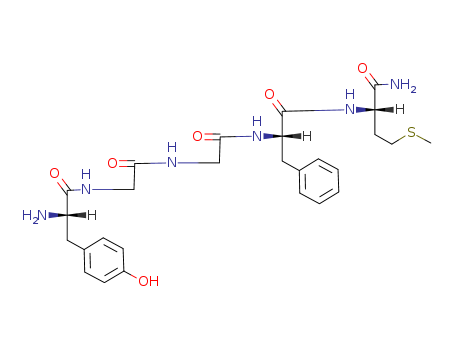
Met-enkephalinamide is a naturally occurring opioid peptide and neuromodulator, acting as an agonist at μ-opioid receptors (μORs) and δ-opioid receptors (δORs). This pentapeptide, composed of five amino acids - methionine, enkephalin, and amide, functions as a neurotransmitter in both the central and peripheral nervous systems. Recognized for its analgesic properties, Met-enkephalinamide plays a key role in pain relief by binding to opioid receptors in the body. Beyond its analgesic effects, Met-enkephalinamide is involved in regulating various physiological processes, including mood, stress responses, and immune function. Its significance extends to potential applications in the treatment of conditions such as chronic pain, addiction, and mood disorders. As an endogenous opioid peptide, Met-enkephalinamide underscores its importance in the body's natural mechanisms for pain modulation and overall neurophysiological regulation. |
InChI:InChI=1/C27H36N6O6S/c1-40-12-11-21(25(29)37)33-27(39)22(14-17-5-3-2-4-6-17)32-24(36)16-30-23(35)15-31-26(38)20(28)13-18-7-9-19(34)10-8-18/h2-10,20-22,34H,11-16,28H2,1H3,(H2,29,37)(H,30,35)(H,31,38)(H,32,36)(H,33,39)/t20-,21-,22-/m0/s1
Peptide syntheses are performed in vario...
Chemical synthesis of peptides has been ...
The simplicity in the removal of N-prote...
The solid-phase synthesis of several ana...

| Conditions | Yield |
|---|---|
|
With hydrogen fluoride; methoxybenzene; at 0 ℃; for 1h; Yield given;
|
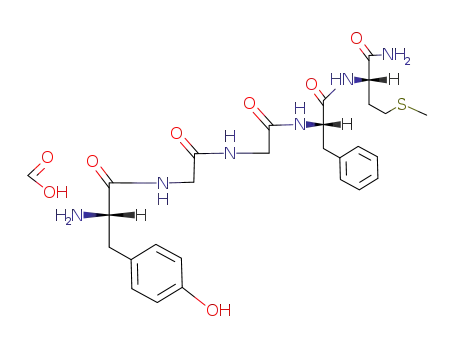
Tyr-Gly-Gly-Phe-Met-NH2*HCOOH

| Conditions | Yield |
|---|---|
|
With sodium hydrogencarbonate; In water; for 0.5h;
|
74% |

Tyr-Gly-Gly-Phe-Met-NH2*HCOOH
CAS:40077-57-4
CAS:47931-85-1
CAS:74-79-3
CAS:83150-76-9
