- 13035986501
- 13035986501
- Nyx-peptide@jsjpharm.cn
Your Location:Home >Products >Custom peptide >83150-76-9

Product Details
|
Indications |
Octreotide acetate (Sandostatin) is a synthetic peptide analogue of the hormone somatostatin. Its actions include inhibition of the pituitary secretion of growth hormone and an inhibition of pancreatic islet cell secretion of insulin and glucagon. Unlike somatostatin, which has a plasma half-life of a few minutes, octreotide has a plasma elimination half-life of 1 to 2 hours. Excretion of the drug is primarily renal. |
|
Therapeutic Function |
Antiulcer, Growth hormone inhibitor |
|
Biological Functions |
Octreotide acetate, a long-acting octapeptide analogue of somatostatin, has a half-life of approximately 100 minutes. A comparison of the primary structures of octreotide and somatostatin suggests little similarity, but from earlier work at the Salk Institute it was known that not all the residues in somatostatin were necessary to elicit its full biological activity. Other studies suggested that the essential fragment for its activity was the tetrapeptide Phe7-Trp8- Lys9-Thr10. These earlier studies helped in the design of the potent drug now known as octreotide acetate. This drug suppresses the secretion of gastroenteropancreatic peptides, such as gastrin, vasoactive intestinal peptide (VIP), insulin, and glucagon, as well as pituitary GH. Furthermore, it is more potent than natural somatostatin in inhibiting the release of glucagon, insulin, and GH. |
|
Biochem/physiol Actions |
Octreotide is three times more potent than the native hormone in inhibiting the secretion of growth hormone glucagon and insulin in vivo. Octreotide regulates serum prolactin levels and resolves galactorrhea or (secondary) amenorrhea in acromegaly patients. Hence, this peptide can be considered as a potent therapeutic for acromegaly treatment. |
|
Veterinary Drugs and Treatments |
Octreotide may be useful in the adjunctive treatment of hyperinsulinemia in patients with insulinomas (especially dogs, ferrets). Response is variable, presumably dependent on whether the tumor cells have receptors for somatostatin. Octreotide may also be useful in the diagnosis and symptomatic treatment of gastrinomas in dogs or cats. It may be of use in the treatment of acute pancreatitis, but more research is needed before it can be recommended for this use in veterinary patients. |
|
Mode of action |
Octreotide acetate is a synthetic somatostatin analogue with similar pharmacologic effects to naturally occurring somatostatin, but with a prolonged duration of action. It inhibits pathologically increased secretion of growth hormone, thyroid stimulating hormone, and serotonin, insulin, glucagon, and other peptides produced within the gastro-entero-pancreatic endocrine system. Somatostatin is cell cycle phase-specific, mediating arrest at the G1- phase. Long acting somatostatin analogues have been shown to inhibit tumour growth. |
|
|
|
|
Brand name |
Sandostatin (Novartis). |
|
General Description |
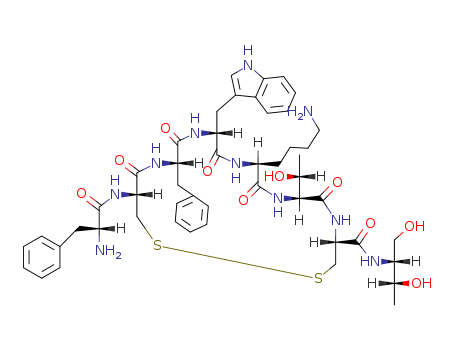
Octreotide is a somatostatin analogue with D-Phe-Cys-Phe-D-Trp-Lys-ThrCys-Thr-OH amino acid sequence. |
InChI:InChI=1/C49H66N10O10S2.C2H4O2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41;1-2(3)4/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64);1H3,(H,3,4)/t28-,29-,34-,36+,37+,38-,39-,40+,41+,42+;/m1./s1
A solid-phase method for the preparation...
Structure–activity relationship studies ...
PROBLEM TO BE SOLVED: To provide a metho...
(Figure Presented) Getting the better of...
The new electrophilic trifluoromethylati...
H-D-Phe-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr-OH

octreotide
| Conditions | Yield |
|---|---|
|
With ammonium acetate; pyrographite; In tetrahydrofuran; water; at 20 ℃;
|
85.6% |
D-Phe-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr(ol)

octreotide
| Conditions | Yield |
|---|---|
|
In water; at 20 ℃; for 47.5h; pH=7.4; aq. phosphate buffer;
|
80% |
|
With phosphate buffer; air; at 25 ℃; for 48h; Yield given;
|
|
|
With ammonium acetate; for 48h; pH=7.0;
|
|
|
With air;
|
Boc-D-Phe-OH
Togni's reagent
Togni's reagent II
boc-octreotide
CAS:40077-57-4
CAS:47931-85-1
CAS:60117-17-1
CAS:196078-30-5
