- 13035986501
- 13035986501
- Nyx-peptide@jsjpharm.cn
Your Location:Home >Products >Amino acid >74-79-3

Product Details
|
Indications and Usage |
Odorless, slightly bitter. Easily soluble in water (solubility in 0℃ water is 83g/L, solubility in 50℃ water is 400g/L), very slightly soluble in ethanol, insoluble in ether; pI6.0; loses its 2-molecule water crystal when heated to 105℃, darkens in color at 230℃, disintegrates at 244℃; its aqueous solution has maximum absorption at 205nm (1gε3.28). L-Arginine is an encoding amino acid in protein synthesis and is one of the 8 essential amino acids in the human body. The body needs it for many different functions. Taking L-Arginine supplements can treat certain diseases such as congestive heart failure and cystitis. L-Arginine can also act as seasoning for nutrient supplements and food additives. L-Arginine can undergo a heat reaction with sugar (amino-carbonyl reaction) to obtain a unique fragrance, GB 2760-2001, an approved food spice. As an amino acid drug, L-Arginine can be used as pharmaceutical raw material and is an important ingredient in amino acid infusions and integrated amino acid preparations. It is also a crucial amino acid in maintaining infant growth and maturation. |
|
Mechanisms of Action |
L-Arginine can stimulate the human body to release certain chemicals such as insulin and human growth hormone. It can also clear ammonia in the body and promote the healing of wounds. The human body also needs it to produce sarcosine. Decomposing L-Arginine produces nitric oxide, which can expand blood vessels and increase blood flow. L-Arginine is an intermediate metabolite in the orthinine cycle and promotes the conversion of ammonia to urea, thus lowering the blood concentration of ammonia. L-Arginine is also an important part of sperm protein and can promote spermatogenesis and provide energy for sperm movement. Additionally, intravenous arginine can stimulate the pituitary to release growth hormone and can be used to test pituitary functions. |
|
Adverse reactions |
Abdominal pain, diarrhea, gout and bloating. There may also be increased severity in herpes breakouts and increased effects of antihypertensive drugs, resulting in a lower blood pressure than expected, which may cause hypertensive patients to experience dizziness and fainting. |
|
Toxicity Level |
Moderate |
|
Acute Toxicity |
Reference data: abdominal cavity – large rat LD50: 3793 mg/kg. |
|
Flammability Characteristics |
Flammable. Burning produces toxic nitrogen oxide smoke. |
|
Handling |
Store in ventilated, cool and dry area. |
|
Extinguishers |
Dry powder, foam, sand, carbon dioxide, water mist. |
|
Chemical Properties |
Arginine is a diaminomonocarboxylic acid. The nonessential amino acid, arginine, is a urea cycle amino acid and a precursor for the neurotransmitter nitric oxide, which plays a role in the regulation of the brain’s system of dilation and constriction of small blood vessels. It is strongly alkaline and its water solutions absorb carbon dioxide from the air (FCC, 1996). Functionality in foods includes, but is not limited to, nutrient and dietary supplement |
|
Occurrence |
Reported present in cheese, chocolate, eggs, meat, nuts and other products. |
|
Uses |
L-Arginine is used for heart and blood vessel conditions which includes congestive heart failure (CHF), chest pain, high blood pressure and coronary artery disease. It plays a vital role in the treatment of cardiovascular disease due to it being antiatherogenic, anti-ischemic, antiplatelet and antithrombotic. It acts as a growth stimulant and is involved in the treatment of erectile dysfunction in men. It is an important ingredient of tooth paste which provides effective relief for sensitive teeth. |
|
Definition |
ChEBI: An L-alpha-amino acid that is the L-isomer of arginine. |
|
Aroma threshold values |
Detection at 100%, faint. |
|
Taste threshold values |
Taste characteristics at 1000 ppm: hint of sourness. |
|
General Description |
L-Arginine is an amino acid that plays a key role in many physiological processes such as tissue repair and reproduction. It is a key precursor for synthesizing nitric oxide in mammals. Due to these factors, the dietary supplementation with L-arginine may show a range of health benefits. |
|
Biochem/physiol Actions |
Substrate of nitric oxide synthase, which is converted to citrulline and nitric oxide (NO). Induces insulin release by a nitric oxide-dependent mechanism. |
|
Safety Profile |
Mutation data reported. Whenheated to decomposition it emits toxic fumes of NOx. |
|
Synthesis |
Enzymatically, arginine is formed in two reactions from citrulline. The first reaction (citrulline + succinate) is catalyzed by the enzyme arginosuccinate synthetase. It is ATP dependent and with the formation of a new C–N bond in the gaunidino group of arginosuccinate, water is removed and ATP is hydrolyzed. The second reaction is catalyzed by arginine synthetase and involves the scission of arginosuccinate with the formation of arginine and fumaric acid. |
|
Purification Methods |
S-Arginine crystallises from H2O as the dihydrate and as plates from EtOH. It also crystallises from 66% EtOH. Its solubility in H2O is 15% at 21o. Its isoelectric point is at pH 10.76. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 1841 1961, Beilstein 4 IV 817.] |
InChI:InChI=1/C6H14N4O2/c7-4(5(11)12)2-1-3-10-6(8)9/h4H,1-3,7H2,(H,11,12)(H4,8,9,10)/p+1/t4-/m0/s1
Methylated free amino acids are an impor...
Amino acids are key synthetic building b...
Guanidine prenylation is an outstanding ...
Proteolysis of proteins and peptides is ...
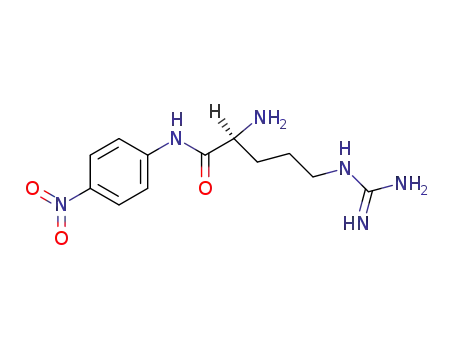
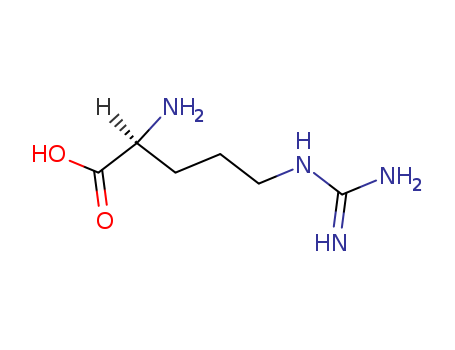
H-Arg-pNA

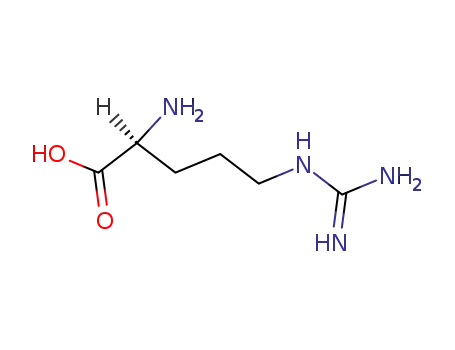
L-arginine

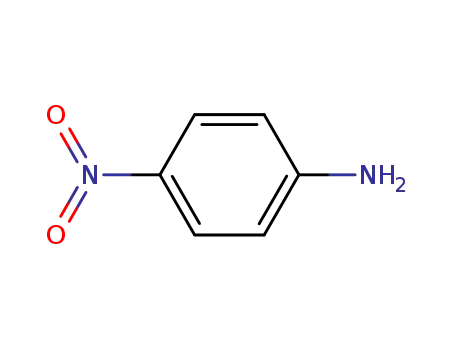
4-nitro-aniline
| Conditions | Yield |
|---|---|
|
With Tris-HCl buffer; an aminopeptidase from the seeds of Cannabis sativa; water; at 37 ℃; pH=7.5; Enzyme kinetics; Enzymatic reaction;
|
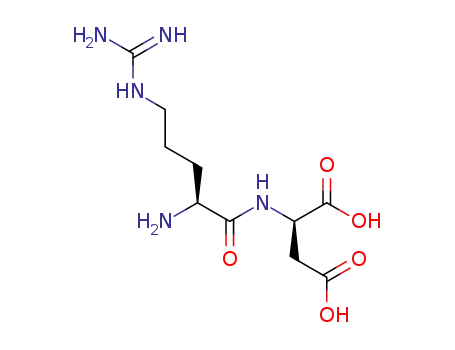
L-Arg-D-Asp

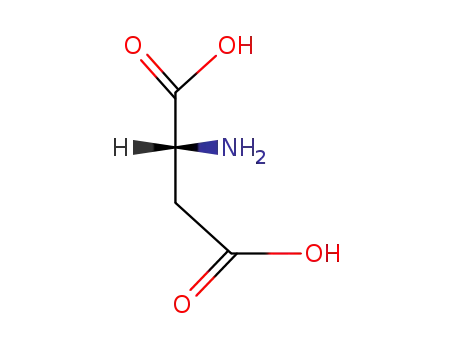
(2R)-aspartic acid


L-arginine
| Conditions | Yield |
|---|---|
|
With recombinant Streptomyces coelicolor Sco3058 dipeptidase; water; at 30 ℃; pH=8; Concentration; Kinetics; aq. buffer; Enzymatic reaction;
|
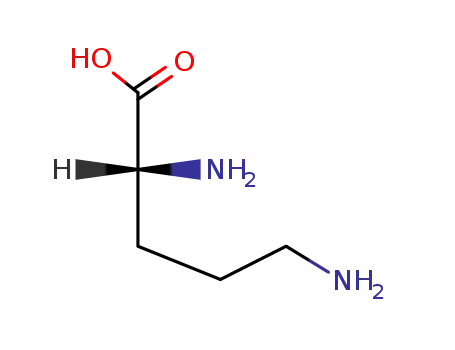
D-ornithine
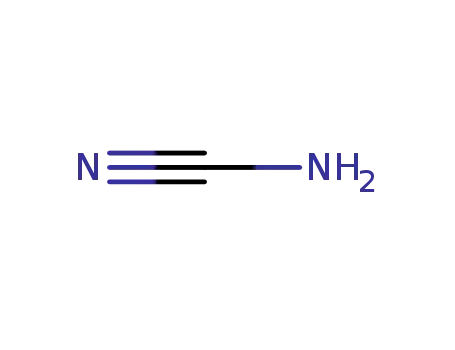
CYANAMID
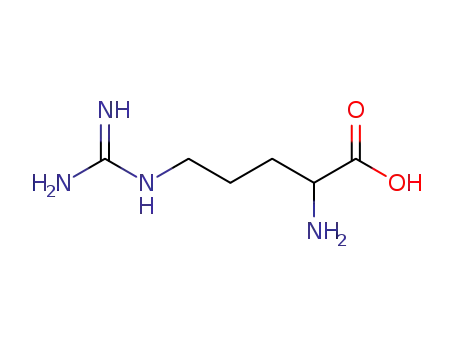
Arg
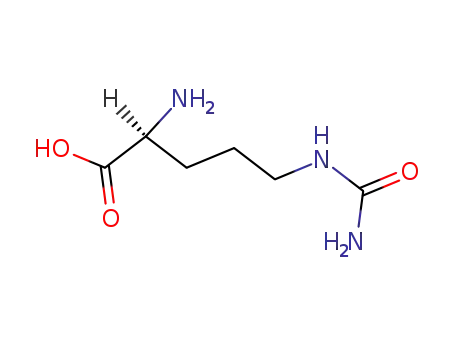
Citrulline
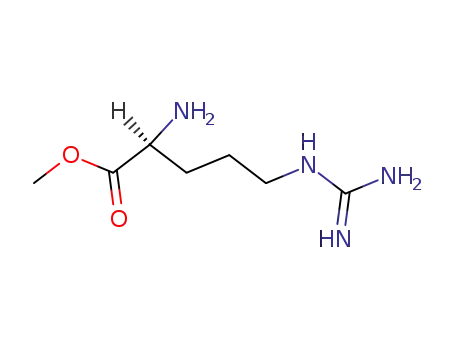
L-arginine methyl ester
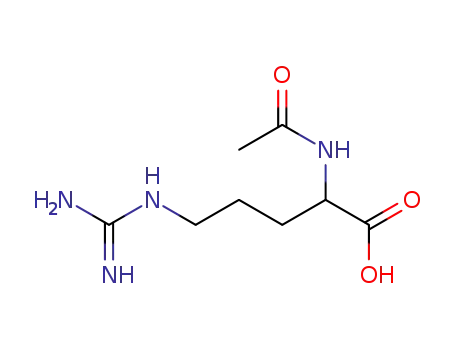
Nα-acetylarginine
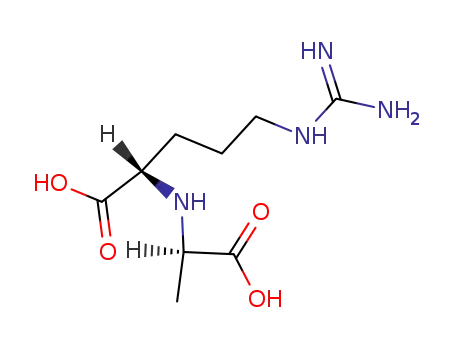
N2-(D-1-carboxyethyl)-L-arginine
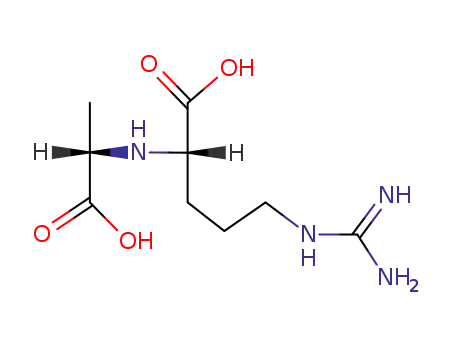
(S),(S)-octopine
CAS:247062-33-5
CAS:79561-22-1
CAS:58569-55-4
CAS:60117-17-1
