- 13035986501
- 13035986501
- Nyx-peptide@jsjpharm.cn
Your Location:Home >Products >Custom peptide >320367-13-3

Product Details
|
Description |
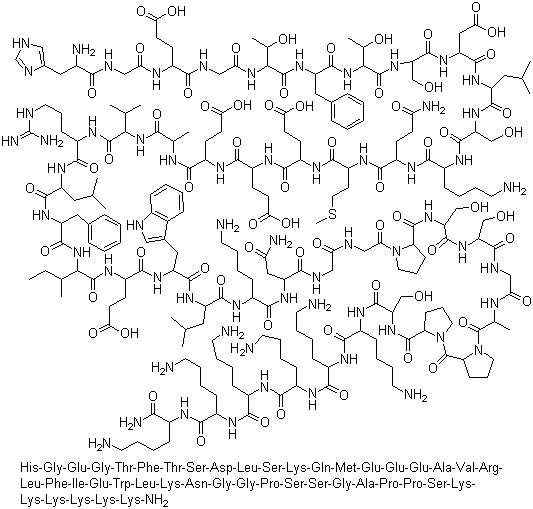
Lixisenatide is a once-daily injectable GLP-1 receptor agonist used for the treatment of type 2 diabetes. It is marketed as Lyxumia in the European Union and Adlyxin in the U.S., manufactured by Sanofi. The medication is administered as an adjunct to diet and exercise to manage type 2 diabetes. In the European Union, it is specifically employed to complement insulin therapy. |
|
Uses |
Lixisenatide is a 44-amino acid exendin-4-like analogue, modified at the C-terminal with the addition of six lysine residues and the deletion of one proline. Its mechanism of action involves stimulating the GLP-1 receptors, helping regulate blood sugar levels in individuals with diabetes. The impact of lixisenatide on a person's risk of death is uncertain as of 2017. The injection is part of a comprehensive approach to treating type 2 diabetes, where the body struggles to use insulin effectively, leading to difficulties in controlling blood sugar levels. |
Type 2 diabetes mellitus (T2DM) is an increasing health problem worldwide. Glucagon-like peptide-1 (GLP-1) receptor agonists are an expanding drug class that target several of the pathophysiological traits of T2DM. Lixisenatide is a GLP-1 receptor agonist in development for once-daily treatment of T2DM.
Cardiovascular morbidity and mortality are higher among patients with type 2 diabetes, particularly those with concomitant cardiovascular diseases, than in most other populations. We assessed the effects of lixisenatide, a glucagon-like peptide 1–receptor agonist, on cardiovascular outcomes in patients with type 2 diabetes who had had a recent acute coronary event.
CAS:40077-57-4
CAS:47931-85-1
CAS:108736-35-2
CAS:58569-55-4
